Handbook of the Mammals of the WorldVolume 1: Carnivores
Original price was: 160.00€.136.00€Current price is: 136.00€.
Illustrator
In stock
Original price was: 160.00€.136.00€Current price is: 136.00€.
Weight
4.35 kg
Size
24 × 31 cm
Language
English
Format
Hardback
Pages
728
Publishing date
May 2009
Published by
Lynx Edicions
Description
Volume 1: Carnivores is the inaugural volume of the Handbook of the Mammals of the World (HMW) series, and it sets a high standard for the series with its comprehensive coverage of the Order Carnivora.
This volume features in-depth coverage of all 13 carnivore families, showcasing the diversity, biology, and conservation status of over 245 species, including some of the planet’s most iconic and threatened animals.
The volume begins with an overview of the evolutionary history, morphology, and adaptive strategies of the Carnivora. It includes detailed descriptions of each carnivore family, from the well-known big cats and bears to the smaller but equally intriguing families like mustelids, mongooses, and civets. Each species account features up-to-date information on distribution, habitat, behavior, diet, and reproduction, alongside high-quality illustrations and distribution maps. The systematics of each family are thoroughly reviewed, incorporating the latest scientific research on taxonomy and evolutionary relationships.
This first volume of the series sets the bar for the comprehensive and accessible treatment of the world’s mammals. With contributions from leading experts in the field and the trademark quality of Lynx Edicions, Volume 1: Carnivores is an indispensable resource for researchers, wildlife enthusiasts, conservationists, and anyone with a passion for the natural world.
Order CARNIVORA
| Family Nandiniidae (African Palm Civet) | Philippe Gaubert |
| Family Felidae (Cats) | Mel E. Sunquist & Fiona C. Sunquist |
| Family Prionodontidae (Linsangs) | Philippe Gaubert |
| Family Viverridae (Civets, Genets and Oyans) | Andrew P. Jennings & Geraldine Veron |
| Family Hyaenidae (Hyenas) | Kay E. Holekamp & Joseph M. Kolowski |
| Family Herpestidae (Mongooses) | Jason S. Gilchrist, Andrew P. Jennings, Geraldine Veron, & Paolo Cavallini (Coordinator) |
| Family Eupleridae (Madagascar Carnivores) | Steven M. Goodman |
| Family Canidae (Dogs) | Claudio Sillero-Zubiri |
| Family Ursidae (Bears) | David L. Garshelis |
| Family Ailuridae (Red Panda) | Fuwen Wei & Zejun Zhang |
| Family Procyonidae (Raccoons) | Roland Kays |
| Family Mephitidae (Skunks) | Jerry W. Dragoo |
| Family Mustelidae (Weasels and relatives) | Serge Larivière & Andrew P. Jennings |
The volume does not include marine carnivores like seals and sea lions, as these are featured in Volume 4: Sea Mammals.
- 36 colour plates
- 561 colour photographs
- 258 distribution maps










































 Copyright 2025 © Lynx Nature Books
Copyright 2025 © Lynx Nature Books
Gehan de Silva Wijeyeratne –
Handbook of The Mammals of the World is inspired by and follows the general format of The Handbook of the Birds of the World. Both series are invaluable references but also testament to the ambition of the human spirit to seek to catalogue living things in the spirit of scientific explorers in the past. The upside is that the modern cataloguers are equipped with modern tools such as molecular genetics. The downside is they are working in a world which is imperilled by habitat loss and climate change which has put many species at risk. Despite the strong scientific underpinnings and the framework it provides for conservation decisions, both handbook series are accessible and attractive.
As with the volumes on birds, in this opening volume of the mammal series, the text on the families and introductory section is very readable to any person and does not require a science background. Most people with an interest in wildlife will find the family sections of absorbing interest. The species accounts are for reasons of brevity and space more telegraphic in style. They also include details, for example on dentition, which non-specialists can gloss over. The book is liberally illustrated with artwork and a fabulous selection of photographs. The photographs have been selected to show interesting aspects of behaviour. Generally, images chosen are technically strong in terms of composition, focus and exposure and make the book a visual feast as well.
Starting the first volume of the mammal series with carnivores is an excellent choice as very few groups of mammals have a more popular appeal. Carnivores are a natural evolutionary grouping with all species having a common ancestor. The contents page lists the 13 families in the order carnivora and the list of plates executed by Toni Llobet. The family accounts have been written by 17 experts who are specialists on certain families.
Most of the 13 families are familiar. They comprise African Palm Civet, Cats, Linsangs, Civets, Hyenas, Mongooses, Madagascar Carnivores, Dogs, Bears, Red Panda, Racoons, Skunks and Weasels. For the more scientifically inclined, page 12 contains a tabulation at a more granular level using a modern molecular phylogenetic arrangement. This breaks down the order Carnivora into two Suborders, Feliformia and Caniformia. Feliformia includes the families from Cats to Madagascar Carnivores and Caniformia includes the rest from Dogs to Weasels and relatives. Eight of the families are in turn divided into subfamilies. For example, the Family Felidae is divided into the subfamilies Pantherinae and Felinae. The subfamily Pantherinae comprises two genera, Neofelis and Panthera, the latter containing familiar and popular species such as the Tiger. The subfamily Felinae comprises 12 genera, holding the bulk of the species of cats.
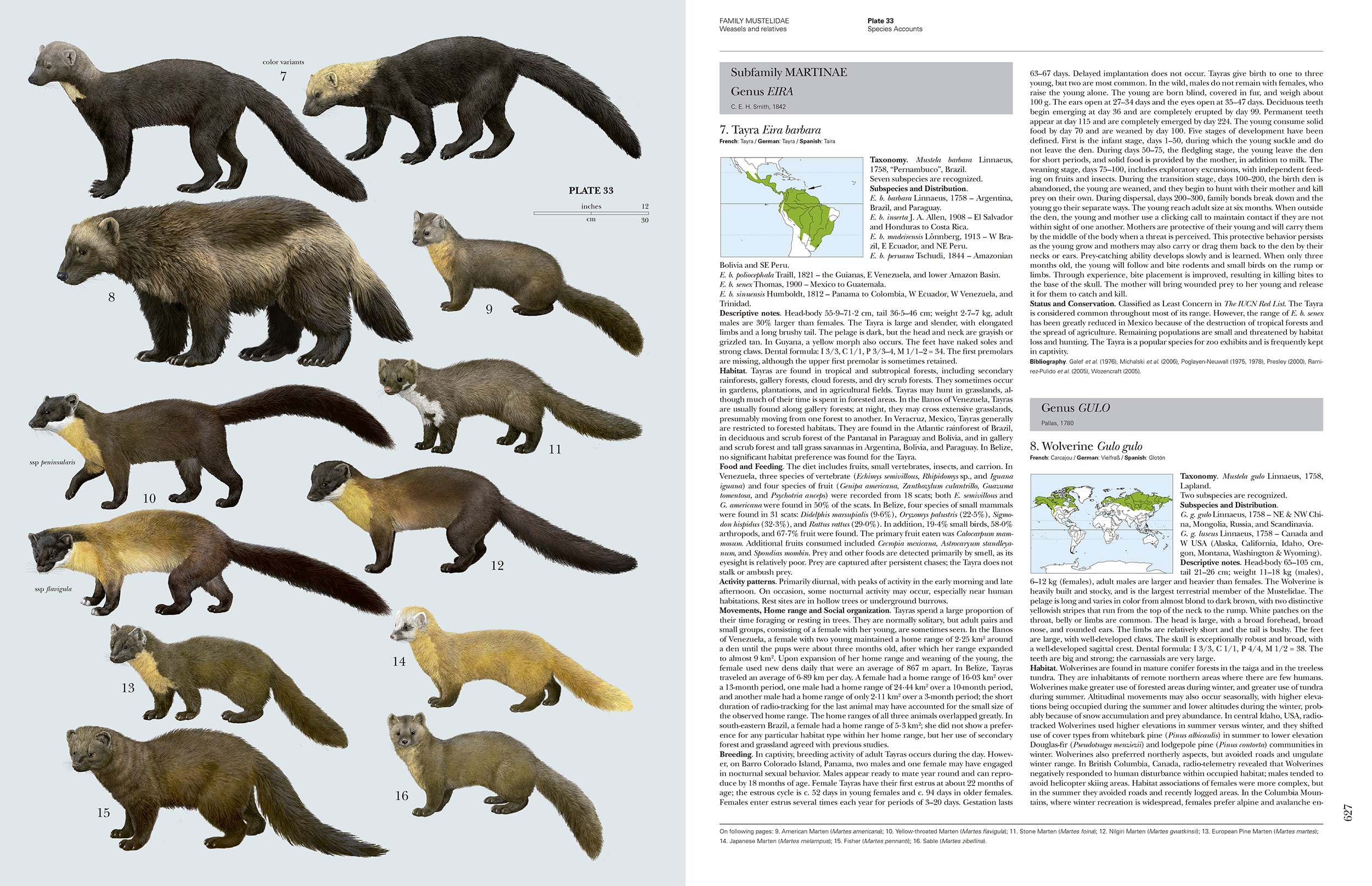
The book although written to work as a single coherent volume is akin to a series of family monographs written by subject matter experts. One of the strengths of the book is the family introduction which is wide in scope and written in an accessible manner to bring together what has been published in hundreds of scientific papers. The bibliography for the entire volume contains more than 4,000 references. The family introductions provide an illuminating portrait at family level. The page count allocated is generous. For example, the section Felidae (cats) runs to 72 pages (pages 54 to 125). The family section follows a standard format covering topics such as Systematics, Morphological Aspects, Habitat, Communication, Food and Feeding, Breeding, Movements, Home Range and Social Organisation, Relationship with Humans and Status and Conservation. The systematics section is well covered given the state of change in classification based on molecular work. In some families simplified phylogenetic diagrams are included, at times together with other linear diagrams with pictures to show the evolutionary relationships. This is one of the sections I like as I want to understand how species fit together. This also interests me as I have written and photographed an identification guide to the mammals of Sri Lanka and I need to keep abreast of taxonomic changes, although sometimes for ease of use, like many authors I may retain an older taxonomy. I appreciate other readers may find some of the other family topics more interesting, such the section on social organisation, which brings together fascinating information based on field work by many scientists.
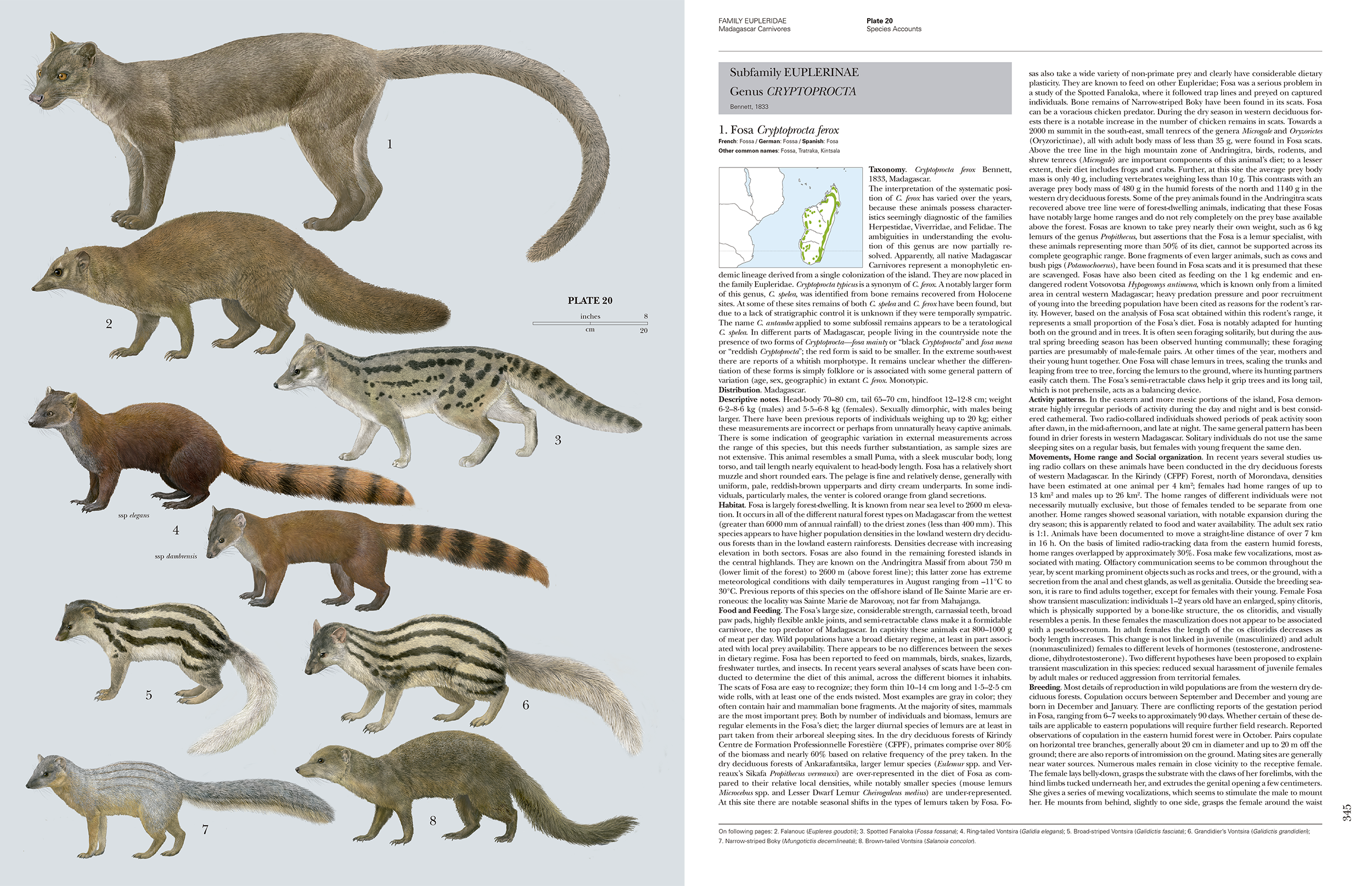
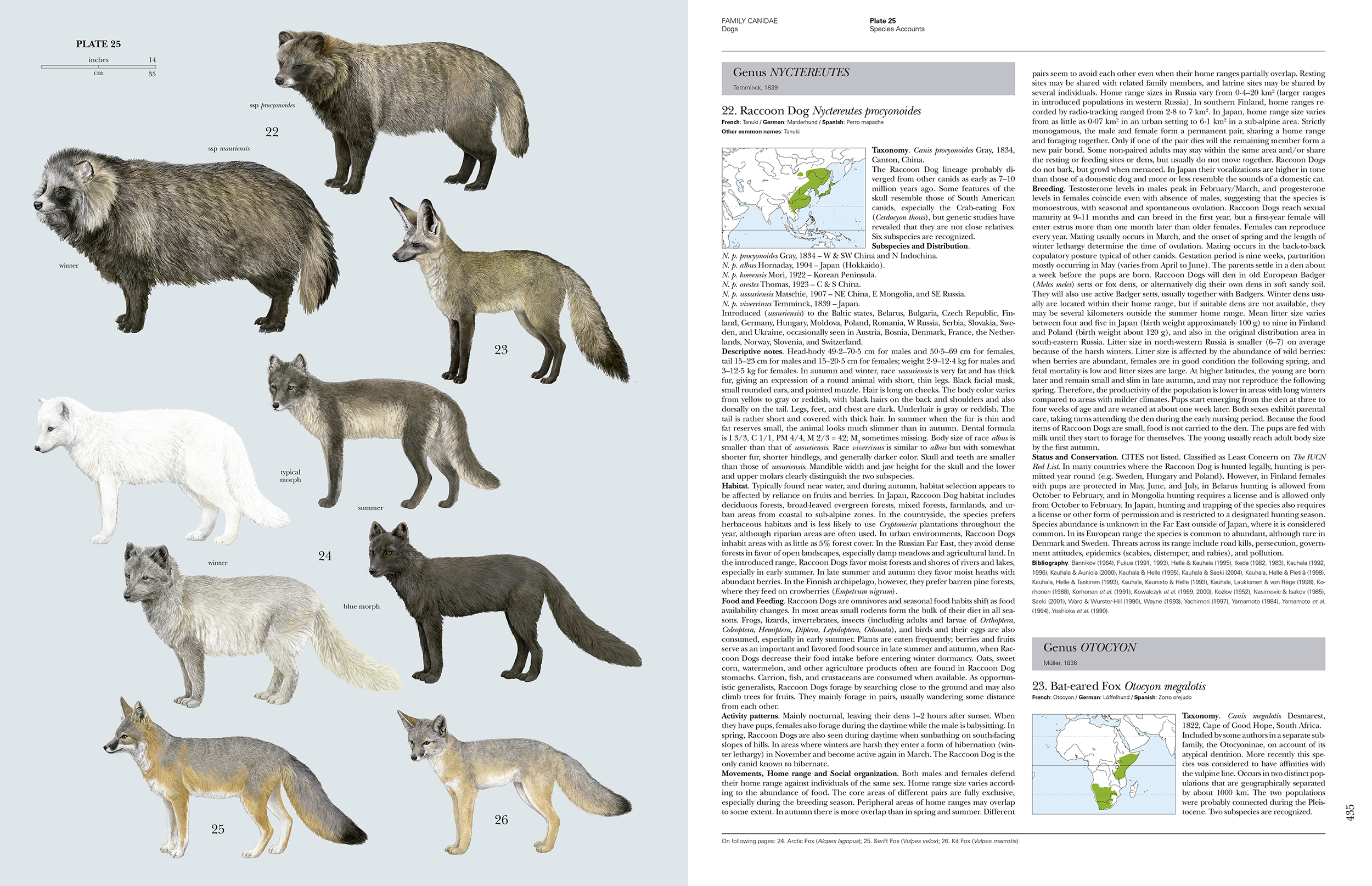
Each family section is followed by the species accounts, which comprise a series of plates with the species sequentially numbered on the plates followed by the text which has the same sequential number. A plate is the leading page for a cluster of text pages that describe the species on that plate. This makes navigation fairly easy within what is a bulky book. For the cats, the species sections run from page 126 to 168 covering 37 species with 9 plates. The 36 plates are all executed to a high technical standard and the animals look quite lifelike. There is a photo-realism quality to them. These are some of the best mammal plates I have seen and are consistent in quality as they have all been drawn by Toni Llobet. To be drawn by a single artist is an impressive body of work. I can see the plates from the completed series being used for country level field guides in the future. Each of the species accounts pages also includes the corresponding plate number in the page header to make it easy to find a species account. The genera with the text are also demarcated by large grey shaded boxes. These are a few examples of the thoughtful design considerations to make the book more navigable.
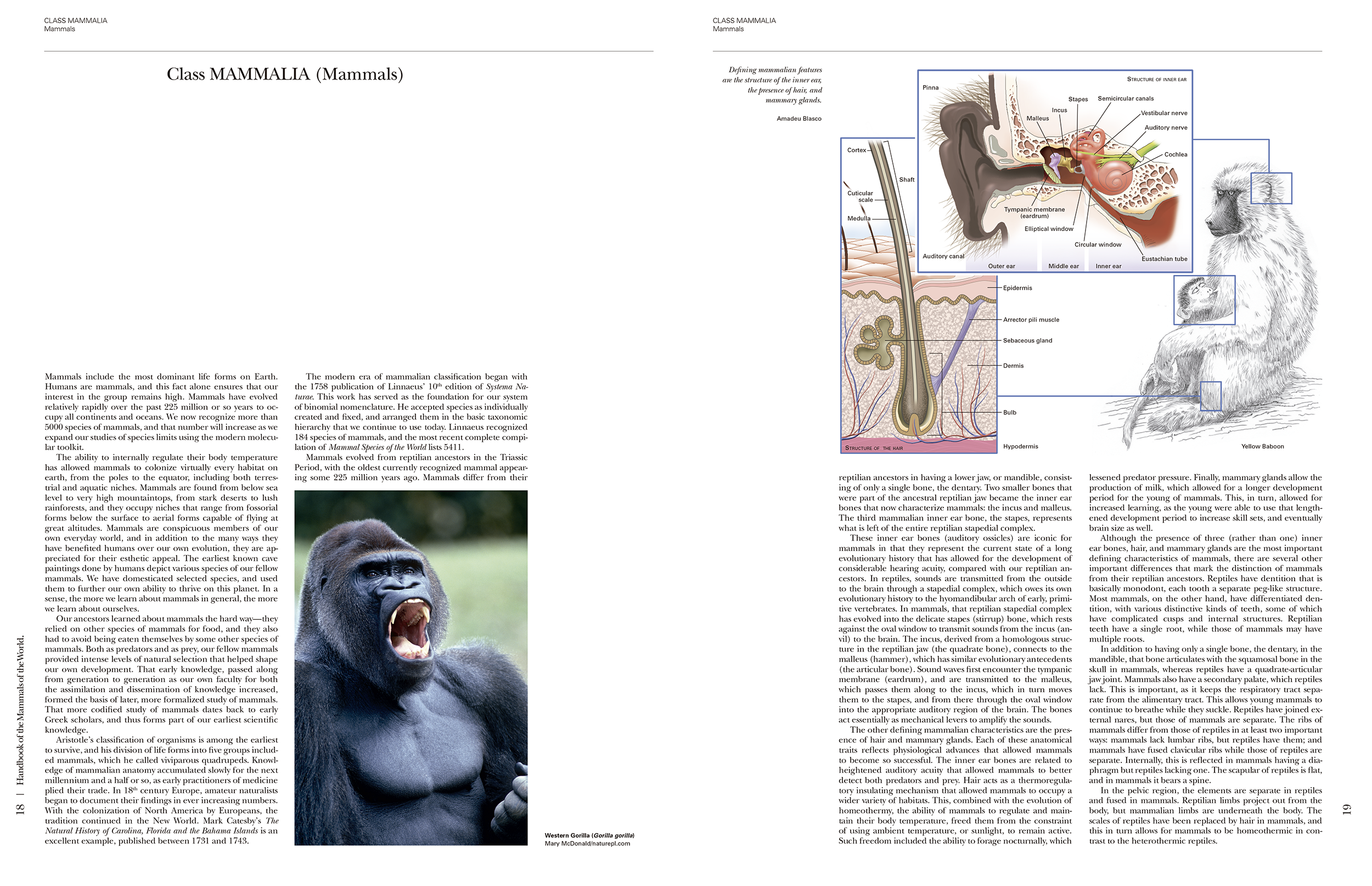
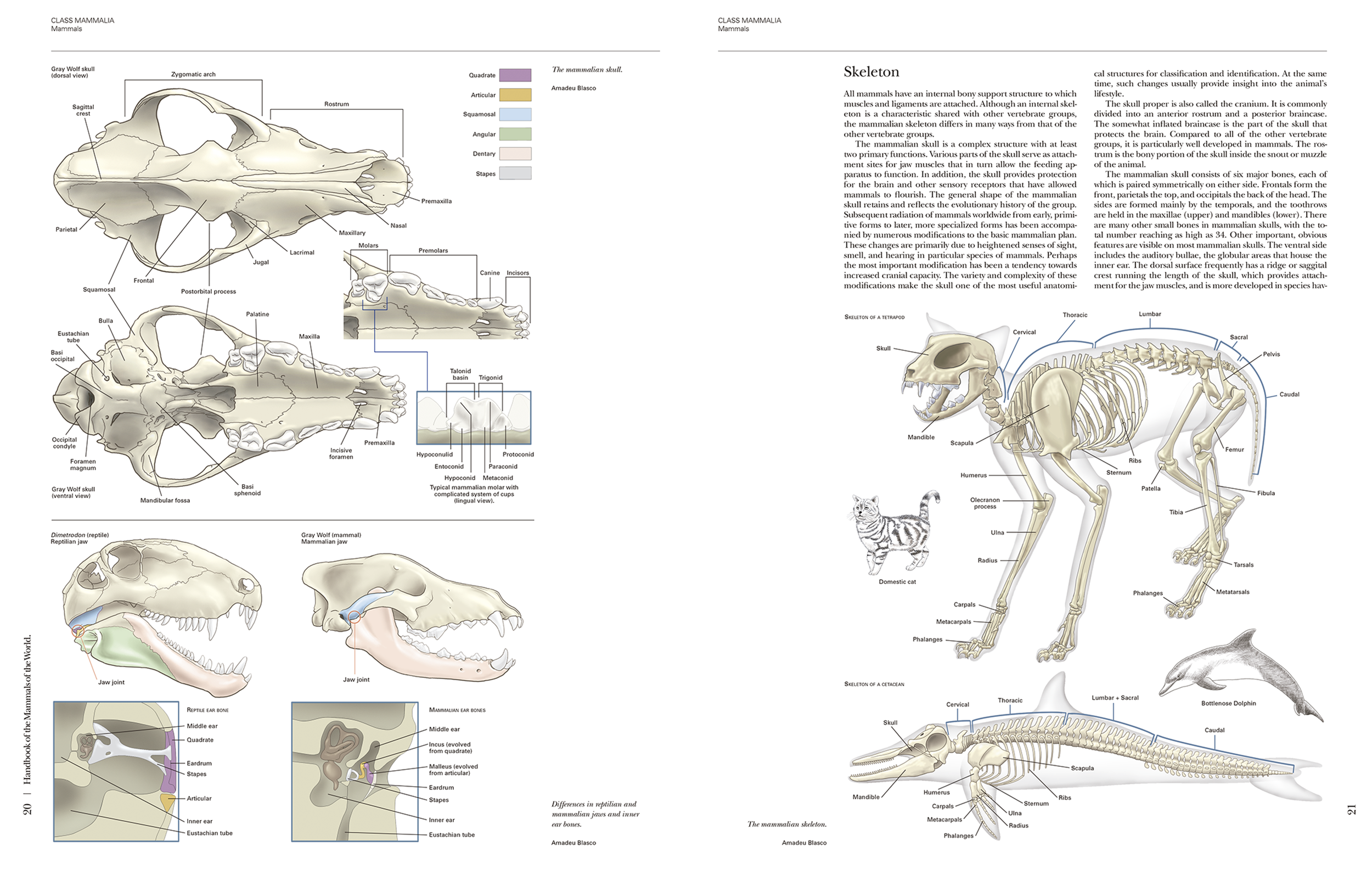
The book begins with an overview of the Class Mammalia, spanning (pages 18-47). This section is liberally illustrated with large diagrams including a cross section of the skin, skeletons, digestive and respiratory structures, circulation and nervous system. Although it looks like something that belongs in a biology text book, it is written in the same accessible style and is a useful read with a lot of information which mammal enthusiasts would find interesting. For example, it explains how desert adapted mammals, use air cooling and use mucus in their nose to recapture water. Or you learn that a mouse spends twenty-five times as much energy foraging as a similar sized lizard; a consequence of maintaining an elevated body temperature. Each of the 29 topics in this section has interesting nuggets. The section ends with a brief explanation on speciation, phylogeny and classification with a useful table summarising numbers of genera and species across orders and families. A pie chart on the previous page shows how the rodents and bats make up more than half of all mammal species.
The end section comprises the massive list of references (pages 659-716) and the index.
This series as with the bird series works in a way that it is made up of two components for each family, the family description and the species accounts. The species accounts and the accompanying plates lend themselves to being re-packaged in the future to make field guides (in various permutations at the level of scientific orders or geographical units such as countries). The species accounts are mainly for reference and not something that an average person would read through. However, I anticipate people will dip into the species accounts that interest them. They are not without some surprises (unless you have been following the scientific literature). For example, with the Tiger the text states that there is little evidence for subspecies differentiation in based on recent analyses of morphological and genetic variation. The text notes the five extant subspecies that are currently recognised. With the Leopard the text notes that recent analyses suggest that the twenty four subspecies that are currently recognised and could subsumed into just three subspecies, one each for Africa, Central Asia and the Indian subcontinent. Each species account has a distribution map, but not to the level of subspecies, perhaps because the subspecies still need to be resolved satisfactorily. The authors have done well to abstract to a page or two in a standard format what is known of a species. The references listed at the end of each species accounts will be useful pointers. The species text is in a slightly smaller font and appears to be more densely packed, but contains much of interest when you look up a species of interest.
The parts of the volume with the introductory sections and the family accounts are very interesting and in an easy to read style. If a page which is not a species account is randomly chosen, for most mammal enthusiasts it will be one which can be pleasurably read. If there was an easy way to detach sections, I can see people who have a long train journey for example on an office commute reading the family sections in small pieces and docking them back again into the book. Well, with printed books you cannot detach and dock sections. So I guess we will need to wait for a version that can be read on an electronic reader such as Kindle. Carnivores have been a good choice to start this series as they are of such interest and so charismatic that they lend themselves readily to television documentaries. Besides that, owning Volume 1 does leave one with the sense that you are owning the first volume of a landmark series in natural history.