Handbook of the Mammals of the WorldVolume 2: Hoofed Mammals
Original price was: 160.00€.136.00€Current price is: 136.00€.
Illustrator
In stock
Original price was: 160.00€.136.00€Current price is: 136.00€.
Weight
4.78 kg
Size
24 × 31 cm
Language
English
Format
Hardback
Pages
886
Publishing date
August 2011
Published by
Lynx Edicions
Description
The species accounts supply complete and up-to-date information at a time when new and increasingly sophisticated methods of DNA analysis are reshaping our knowledge of these species; to give just one example, the family Bovidae has almost doubled its size in the last five years, to the 279 distinct species known today.
- Find out more about the contents of Volume 2 by reading the introduction.
| Families covered in this volume | |
| Family Orycteropodidae (Aardvark) | William Andrew Taylor |
| Family Procaviidae (Hyraxes) | Hendrik Hoeck |
| Family Elephantidae (Elephants) | George Wittemyer |
| Family Manidae (Pangolins) | Philippe Gaubert |
| Family Equidae (Horses and relatives) | Daniel Rubenstein |
| Family Rhinocerotidae (Rhinoceroses) | Eric Dinerstein |
| Family Tapiridae (Tapirs) | Emília Patrícia Medici |
| Family Camelidae (Camels) | William Franklin |
| Family Suidae (Pigs) | Erik Meijaard, Jean-Pierre d’Huart & William Oliver |
| Family Tayassuidae (Peccaries) | Andrew Taber, Mariana Altrichter, Harald Beck & Jaime Gongora |
| Family Hippopotamidae (Hippopotamuses) | Rebecca Lewison |
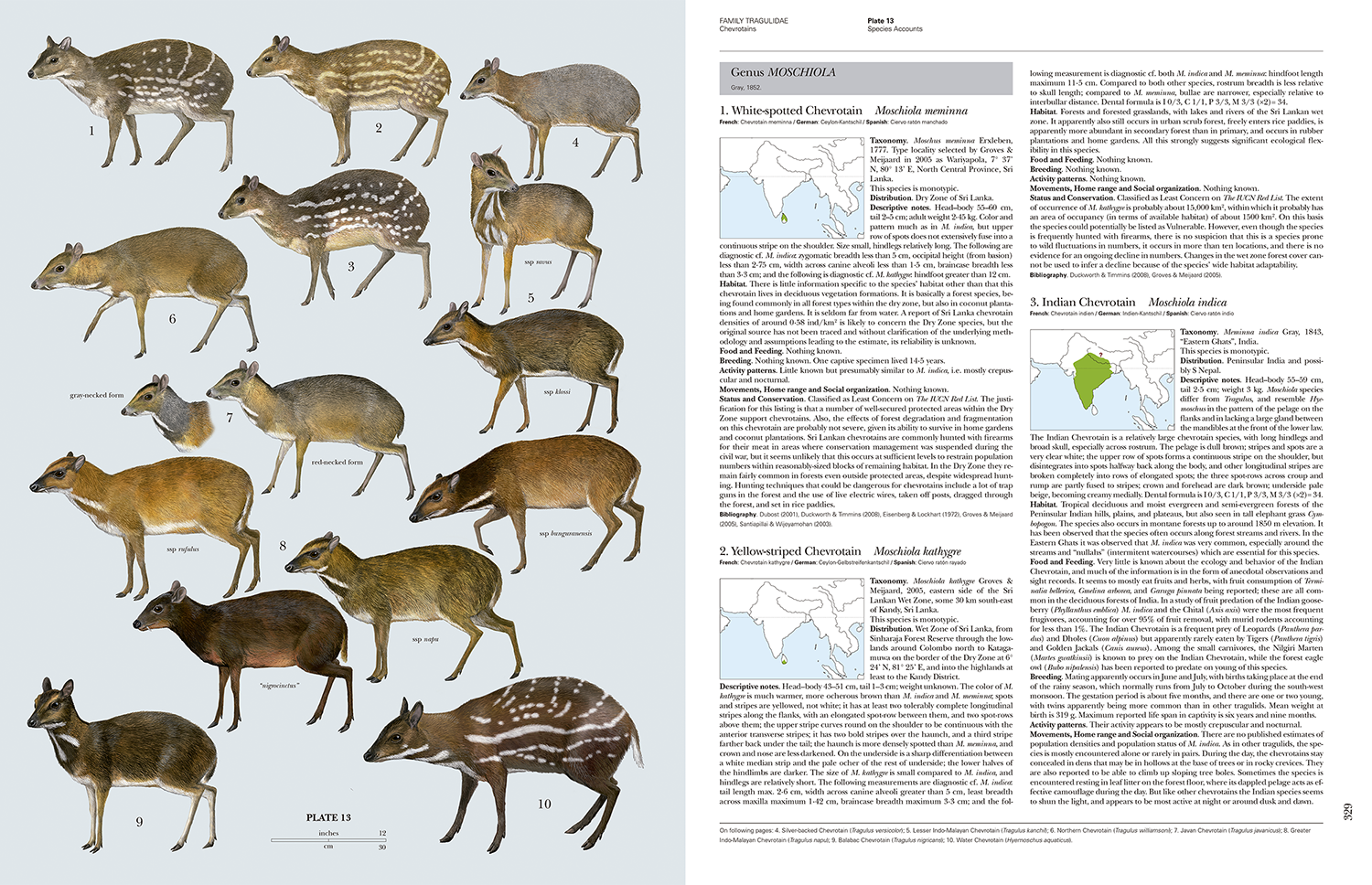
| Family Tragulidae (Chevrotains) | Erik Meijaard |
| Family Moschidae (Musk-deer) | Colin Groves |
| Family Cervidae (Deer) | Stefano Mattioli |
| Family Bovidae (Hollow-horned Ruminants) | Colin Groves, David Leslie, Brent Huffman, Raul Valdez, Khushal Habibi, Paul Weinberg, James Burton, Peter Jarman & William Robichaud |
| Family Antilocapridae (Pronghorn) | John Byers |
| Family Giraffidae (Giraffe and Okapi) | John Skinner & Graham Mitchell |
- 56 colour plates
- 664 colour photographs
- 430 distribution maps
- c.5000 bibliographical references






























































 Copyright 2025 © Lynx Nature Books
Copyright 2025 © Lynx Nature Books
Sem Broeke –
it’s a great book for all lovers of these wonderful animals. It shoes all of the hoofed animals, not just the famous ones. It also shoes some lesser known animals like the pangolins, the hyraxes and the aardvark. In the past five years the bovid species had doubled, because of new DNA research. Althow the book was published in 2011, it contains the newest information from that year about all of the 279 bovid species.
It’s a great book, I defenitely recommend it!
Gehan de Silva Wijeyeratne –
The mammals classified as Hoofed Mammals include some of the most familiar, economically important and iconic mammals in the world. The familiar and economically important sheep, cattle and horses and the iconic elephants are amongst them. These mammals also have a long historical association with people. They can also strike fear and awe. If you have ever been at the receiving end of a charge by a buffalo or elephant you will understand this well. Admittedly ‘Hoofed Mammals’ would not be the choice of label if a PR agency was employed to inject some glamour. But there is no doubting their importance. Horses played a role in the conquest of the New World. Hannibal used elephants to cross the Alps. Today, the multibillion dollar wildlife tourism industry in Africa uses images of elephants, zebras and antelopes in eye-catching advertisements to entice travellers. For the serious wildlife enthusiast who wants a lot of useful information brought together into a single book, Volume 2 in the Handbook of the Mammals of the World is an invaluable reference. Like every volume in the series, it is an Aladdin’s Cave of information.
It is a multi-author work; an international collaboration with 29 authors. Each volume is a Herculean task brought together by a sizeable editorial and administrative team. The completion of the series will mark a significant milestone in the history of zoological literature. Skilful editing has resulted in a book that reads as a coherent whole and the book follows the general format set for the series with the first volume.
Each scientific order of mammals is preceded by an introduction which begins with a text box that has summary information such as the number of genera and species. A useful map allows a quick visual overview of the distribution of the mammals. For example, it is easy to see that the Aardvark, an order with just one species, is widely distributed across Sub-Saharan Africa. The introduction follows standard headings such as Systematics, Morphological Aspects, Habitat, General Habits, Communication, Food and Feeding, Movements, Home Range and Social Organisation, Relationship with Humans, Status and Conservation followed by a general bibliography. They vary in length and not surprisingly, families such as elephants have a longer introduction at 25 pages. These introductions are very well written and will be an absorbing introduction to anyone travelling out of their own country and encountering a family of mammals that is new to them. The family introductions are densely packed with information but yet written in an accessible style and do not come across as text book English. The introduction is liberally illustrated with stunning images and captioned intelligently. One of my favourite images is that of the tip of the trunk of an Asian Elephant grasping a cluster of leaves with the caption explaining it as one of the most sensitive organs found in a mammal. I have spent hundreds of hours photographing elephants and I have never managed to capture an image that shows this so well. The excellent family introductions are followed by the species accounts which follow a field guide style with text facing illustrated plates. The text accompanying the ‘plates’ is in smaller font and the text is more telegraphic in style and intended for reference. The text follows a standard style beginning with Taxonomy, followed by other sections such as Distribution, Habitat, Movements, Home Range and Social Organisation. These sections will differ in appeal to people. I have a preference for understanding the current taxonomy as I write popular field guides and I also like to read on behaviour so that I can interpret what I see in the field. Others may find the Status and Conservation sections, for example, of more interest.
The volume covers six orders of mammals. The Aardvarks (1 species), Hyraxes (5 species), Elephants (3 species) and Pangolins (8 species) are orders with just one family within the order. Therefore, four of the six orders comprise a mere 17 species. However, the two orders Perissodactyla and Artiodactyla include a significant number of species making up the bulk of the book (pages 82 to 809). The perissodactyls or odd-toed ungulates comprise three families. The horses, rhinoceroses and the tapirs. The artiodactyls or even-toed ungulates comprise 9 families. These are the camels, pigs, peccaries, hippopotamuses, chevrotains, musk-deer, deer, bovines (Hollow-horned Ruminants), pronghorn, giraffes and okapi. With the exception of families such as the chevrotains, I suspect almost all of these families are known to almost any city dweller who has access to wildlife documentaries on television or has visited a zoo.
The more complex families have schematics which show how they break down. For example, the bovines (or Hollow-horned Ruminants) comprise 279 species in 54 genera. The family Bovidae is subdivided into two subfamilies, the Bovinae and Antilopinae. The subfamily Bovinae has three tribes, bovini (cattle and buffaloes), nilgai and chowsingha (Boselaphini) and bushbucks, kudus and elands (Tragelaphini). The subfamily Antilopinae comprises 9 tribes and includes many species familiar to anyone who has been on safari in the African savannah. However, seemingly similar mammals such as impalas (Aepycerotini) and gazelles (Antilopini) are in different tribes. One thing this book does is challenge perceptions.
As I browse through the book, I cannot help noticing that I am also ticking off in my mind which tribes of mammals I have encountered in the wild on my travels in various continents. Not surprisingly the systematics section which for some orders may only warrant a fraction of page, in the case of the bovines runs into several pages (pages 444 to 465). To enjoy mammals, you do not need to be conversant with their taxonomy. But for anyone who is interested in evolutionary relationships and likes to understand at a high level how different species of mammals fit together, the schematic diagrams and text are interesting.
The book puts the large format (310 x 240mm) to good use with some images adorning a full page. Another stunning example being the Günther’s dik-dik adorning a full page with a raised crest of hairs. I was immediately reminded of the parallel with birds and reptiles that may raise a crest in courtship or threat display. Whether it is to learn that all dik-diks have this crest of hairs or that bovines doze rather than ‘deep-sleep’ to be in a posture that enables digestion, there appears to be something new to learn in every paragraph. The cervids or deer in the family Cervidae are another group that is of interest to many being found in North and South America, Europe and Asia with notable absences in Africa and Australia and New Guinea. Many tourists to the African savannah think antelopes and gazelles are deer. In fact, antelopes and gazelles are in the family of Hollow-horned Ruminants. The 53 species of deer are in 18 genera are in two subfamilies. The Cervinae with two tribes, the muntjacs and Old World deer both and another three tribes within the subfamily Capreolinae. I have taken many images of Axis or Spotted Deer bounding away in national parks in Sri Lanka. Browsing the picture captions of a deer bounding away I gather that deer that bound away fall into the group of ‘saltatorial’ species living in enclosed habitats as opposed to ‘cursorial species’ living in open habitats. I had not thought of grouping deer like that before and one of the pleasures of a book like this is that it provides new ways in which to reflect on field observations. Other images illustrate how some deer use antorbital glands to mark trees and bushes. Wildlife photographers interested in recording behaviour will find plenty of useful insights on what to be alert to in the field.
The 56 plates by Toni Llobet are to a very high standard. They are accurate, capture the jizz and are photo realistic. One can almost feel the fur on the dik-diks. Where necessary, subspecies are illustrated, such as with the Plains Zebra where 6 subspecies are illustrated. The gleaming images are crisp and look like they have been photoshopped out of photographs. When the series is completed the whole collection of plates will represent one of the most ambitious projects in zoological illustration.
The end sections of the book include the references broken into two parts. The References of Scientific Descriptions (pages 805 to 809) is a convenient summary of the publications relating to the original description of species covered in the volume. This is followed by the General List of References (pages 811 to 870). Such is the pace and volume of scientific papers coming out, even for an author specialising in just one family, it would be difficult to keep abreast.
Not all hoofed mammals are as charismatic as elephants, horses and rhinoceroses or emblematic of safari as with zebras and giraffes. But they are a deeply familiar group of mammals with a long historical relationship with humans and a group that is familiar to everyone one from childhood; perhaps even with a troublesome interloper if you are European householder in the countryside whose prized plants are being eaten away by introduced muntjac which have established feral populations. Whatever your take on the group of mammals, this is a fascinating book if you want to learn more than the space constraints allowed in a portable field guide. Although science does not stand still, it is also an important book which serves as a starting point for any serious zoologist to gain an overview on what is already known.